
These include the seasonal betacoronaviruses hCoV-OC43 and hCoV-HKU1 as well as alphacoronaviruses hCoV-NL63 and hCoV-229E, which usually cause mild respiratory symptoms and account for approximately 5–30% of common colds during the winter season ( Zhu et al., 2020 Li et al., 2020). New coronaviruses that infect humans (hCoVs) emerge frequently, and SARS-CoV-2 is the fifth to be discovered in less than two decades, bringing the total up to seven ( Kahn and McIntosh, 2005). However, the ultimate goal should be a pan-coronavirus vaccine that would be able to induce broad immunity and protection against multiple coronaviruses, thereby preparing us for future outbreaks. To anticipate emerging variants, more broadly protective SARS-CoV-2 vaccines are desirable.

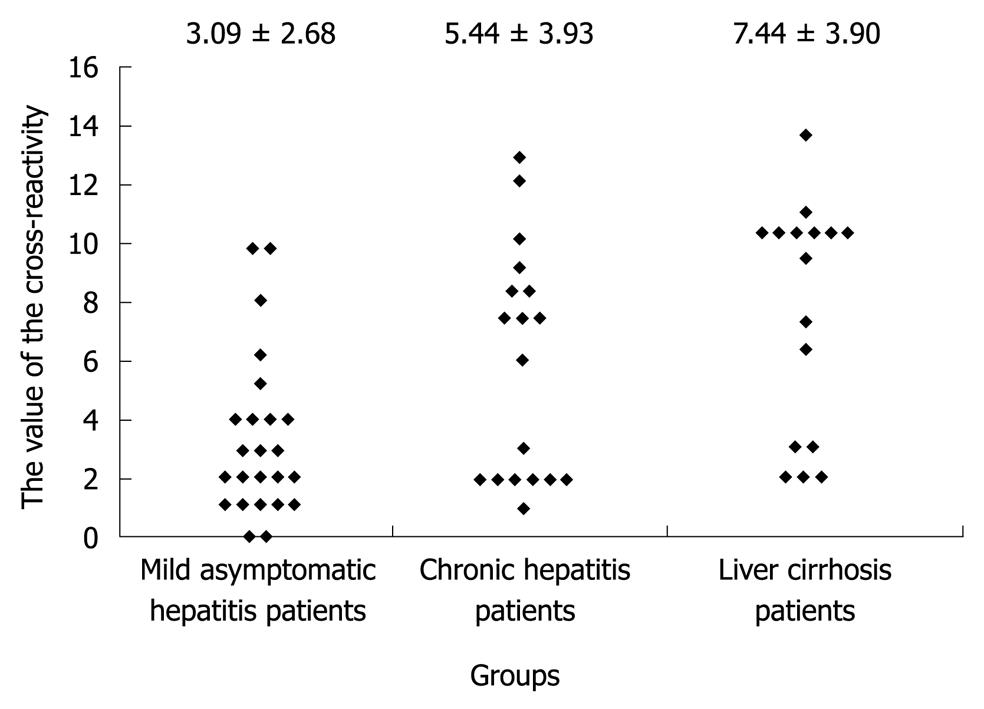
However, these vaccines show reduced efficacy against emerging SARS-CoV-2 variants and vaccine breakthrough infections are frequently reported ( Madhi et al., 2021 Lopez Bernal et al., 2021 Kustin et al., 2021 Hacisuleyman et al., 2021). Following the rapid initiation of many COVID-19 vaccine studies, the first vaccines are already FDA/EMA approved and mass vaccination campaigns are rolled out to hopefully soon subdue this pandemic ( Polack et al., 2020 Baden et al., 2021 Voysey et al., 2021 Sadoff et al., 2021). One and a half year after the emergence of severe acute respiratory coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus infectious disease 2019 (COVID-19), the pandemic is still a major global issue with unprecedented consequences on healthcare systems and economies. These findings support the feasibility of and provide guidance for development of a pan-coronavirus vaccine. In addition, we detected cross-reactive antibodies to all hCoV S proteins after SARS-CoV-2 vaccination in macaques and humans, with higher responses for hCoV more closely related to SARS-CoV-2. We found an 11- to 123-fold increase in antibodies binding to SARS-CoV and MERS-CoV as well as a 2- to 4-fold difference in antibodies binding to seasonal hCoVs in COVID-19 convalescent sera compared to pre-pandemic healthy donors, with the S2 subdomain of the S protein being the main target for cross-reactivity. To inform the development of a pan-coronavirus vaccine, we investigated the presence and specificity of cross-reactive antibodies against the spike (S) proteins of human coronaviruses (hCoV) after SARS-CoV-2 infection and vaccination. Department of Microbiology and Immunology, Weill Medical College of Cornell University, United States Ĭurrent SARS-CoV-2 vaccines are losing efficacy against emerging variants and may not protect against future novel coronavirus outbreaks, emphasizing the need for more broadly protective vaccines.Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Australia.


 0 kommentar(er)
0 kommentar(er)
